Data source
The ABCD Study ( is a prospective, observational, 10-year longitudinal investigation into brain development, spanning from ages 9 to 10 years at baseline through adulthood and comprising 21 study sites34. The study’s rationale and design aspects have been described in detail elsewhere35. Approval for the ABCD Study was given from the central Institutional Review Board at the University of California, San Diego and from the local Institutional Review Boards at University of Maryland, Baltimore, University of Colorado, Boulder, University of Minnesota, the Laureate Institute for Brain Research, Oregon Health and Science University, University of Vermont, University of Pittsburgh, Virginia Commonwealth University, University of Rochester, University of Florida, Medical University of South Carolina, University of Michigan, University of Utah, SRI International, University of Wisconsin-Milwaukee, Children’s Hospital of Los Angeles, Florida International University, Washington University in St. Louis, and Yale University. Data used in these analyses (v5.1; from the 4-year follow-up includes 4754 children and adolescents enrolled between 2016 and 2022, among whom 1234 females had both structural MRI and information on HC use. Specifically, the study includes a total of n = 1169 individuals who did not use HCs (HC−) and n = 65 individuals who reported current HC use (HC + ) and had year 4 follow-up MRI data that passed quality control checks. Analyses with hormone levels included n = 816 in the HC− group and n = 44 in the HC+ group. Parents and guardians provided written informed consent as part of the ABCD study.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Structural brain imaging
All individuals included in this study underwent 3 Tesla structural MRI scans according to standardized protocols36. T1-weighted images were acquired at local study sites, while processing and quality control assessments were conducted at the Data Analysis, Informatics and Resource Center (DAIRC) of the ABCD Study37. FreeSurfer v7.1.1 ( was used, following the ABCD Study’s standardized processing pipeline, to process the images and obtain structural brain measures from 34 regions within each brain hemisphere from the Desikan–Killiany atlas38. Quality control, including both automated procedures and expert visual inspection was performed by DAIRC. For further details on processing and quality control procedures of raw and processed data, see Hager et al.37.
Endocrine assessments, reliability, data selection and cleaning methods
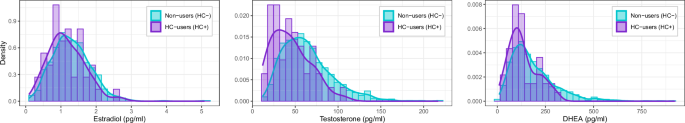
Hormone levels of estradiol, testosterone, and DHEA were measured from saliva samples. Analyses were conducted by Salimetrics ( Estradiol was assessed using the Salimetrics® 17β-Estradiol Enzyme Immunoassay Kit (assay range: 1 pg/ml – 32 pg/ml; assay sensitivity 0.1 pg/ml; serum-saliva correlation: 0.80; intra-assay precision, ≤ 8.1%, inter-assay precision: ≤ 8.9%). Testosterone was assessed using the Salimetrics® Testosterone Enzyme Immunoassay Kit (assay range: 6.1 pg/ml – 600 pg/ml; assay sensitivity 1 pg/ml; serum-saliva correlation: 0.96; intra-assay precision, ≤ 6.7%, inter-assay precision: ≤ 13%). DHEA was assessed using the Salimetrics® DHEA Enzyme Immunoassay Kit (assay range: 10.2 pg/ml – 1000 pg/ml; assay sensitivity 5 pg/ml; serum-saliva correlation: 0.86; intra-assay precision, ≤ 5.8%, inter-assay precision: ≤ 8.5%).
Hormone data were acquired from table ph_y_sal_horm, variable names hormone_scr_dhea_mean (DHEA), hormone_scr_ert_mean (testosterone), and hormone_scr_hse_mean (estradiol). The available hormone data had an unusually high rate of zero values. These could reflect true zeroes (i.e., hormone levels in the participants that were below the limit of detection), or undetectable hormone levels due to errors in sample handling, shipping, processing, analysis, or data management (e.g., erroneously coding missing data as zero). Therefore, based on biologically plausible reference levels, we set a lower boundary for DHEA of 5 pg/mL, for testosterone of 5 pg/mL, and for estradiol of 0 pg/mL. After examining the distribution of data for each hormone, we also removed outliers above 1000, 500, and 1500 pg/mL for each hormone, respectively. Only hormone levels within these boundaries were included in the reported hormone-brain measurement analyses.
To further ensure the reliability of the salivary hormone assessments, we assessed whether time of salivary acquisition (acquired from table ph_y_sal_horm, variable names hormone_sal_start_y [hormone saliva sample time collection started] and hormone_sal_end_y [hormone saliva sample time collection ended]) differed between groups. Time collection did not differ between the two groups (start: t = 0.707, p = 0.456; end: t = 0.816, p = 0.414). Additionally, none of the saliva samples were reported to be contaminated (hormone_sal_notes_y__2), discolored (hormone_sal_notes_y__3), excessively bubbly (hormone_sal_notes_y__4), or insufficient in volume (hormone_sal_notes_y__5).
Hormonal contraceptive use
A total of 1979 adolescents had information available on HC use, measured using a youth survey item which is asked once at each annual visit: Are you currently using hormonal birth control (e.g., the pill, hormone patch, hormone injection)? (variable name menstrualcycle4_y from table ph_y_pds) With response choices “Yes/No”. We elected to use data from youth rather than parent report because of the possibility that the parent or guardian supervising the study visit may be unaware of contraceptive use.
Menstrual staging was not performed in the HC− group. While variables such as menstrualcycle2_y [On average, how many days are there between the first day of your period and the first day of your next period? (e.g., 30 days)] and menstrualcycle3_y [Is your menstrual cycle regular?] provide some information, they are not sufficient for reliable menstrual staging. Given that cycles in adolescents tend to be irregular or anovulatory, it is not feasible to accurately classify participants into follicular or luteal phases based on these self-reports alone. While estradiol levels were available, accurate menstrual staging would require both estradiol and progesterone measurements to distinguish between phases, particularly the luteal phase.
Puberty stage and additional covariates
Puberty stage was determined using the Pubertal Development Scale. We used variable name pds_y_ss_female_category_2 from the latest available wave (Year 4 follow-up). Data used to calculate puberty stages were collected from both parents and youth (variable name: pds_p_ss_female_category_2, pds_y_ss_female_category_2, for parent and youth scales, respectively). We noted that the parent and youth scores had only a moderate correlation (r = 0.285). Because the staging criteria rely on personal and sensitive information (breast growth and onset of menstruation) we elected to use the youth rather than parent report. Age was also considered as covariate. Socioeconomic status was also considered as covariate, which was operationalized using Area Deprivation Index (variable name: reshist_addr1_adi_perc) and family income (variable name: demo_comb_income_v2). Race and ethnicity were also considered as a covariate, which was operationalized using the variable race_ethnicity.
Statistical approach
Statistical analyses were performed using Python (version 3.9.6) with the libraries numpy, pandas, scipy, statsmodels, and scikit-learn. Visualizations were made using the R packages ggplot and ggseg. Demographic variables were compared by Mann–Whitney U test due to non-normality in the underlying distributions, except for race/ethnicity, which was compared by Fisher’s Exact Test. Group differences in hormone levels were evaluated using unpaired t-tests. Group differences in variance in hormone levels were evaluated using Levene’s test to evaluate group-level variance using mean values, and the Brown-Forsythe test to evaluate group-level variance using median values, which is less sensitive to outliers.
In our initial model, effects of HCs on cortical thickness, surface area, and cortical volume were evaluated in a general linear model with group (HC+ vs HC−) as a predictor. Because puberty stage is strongly related to structural brain changes, we tested additional models that included puberty stage and TIV as covariates in the model as well as age and TIV as covariates in a separate model, which did not change the overall pattern of results. Because the general linear model assumes an underlying Gaussian data distribution, we applied the Shapiro-Wilk test for normality and Breusch–Pagan test for homoscedasticity to the residuals. Some ROIs passed and others failed these assumption checks, therefore we also (1) entered the data into a robust regression, and (2) applied a log transformation to normalize the data, then entered into an ordinary least squares model. The general pattern of results was unchanged regardless of the statistical model employed.
Lastly, several approaches were taken to thoroughly interrogate the relationship between brain measures and hormones. We tested hormone-brain measure relationships at the whole group level and also investigated whether the effect of hormones on each brain measurement depended on group status (HC + /HC-). Our initial approach implemented semi-partial Spearman correlations with puberty stage and TIV as covariates. We also evaluated whether the above-reported correlations between hormone levels and brain measures differed between HC+ and HC−. To test this, separate Spearman correlation coefficients were calculated for each hormone-ROI pair for each group and compared using Fisher’s z-test. All analyses were FDR-corrected for multiple comparisons29. Both corrected and uncorrected results are reported.
As a second approach, we fitted LMMs that included interaction terms between group status (HC + /HC−) and each hormone level on each brain measurement (cortical thickness, area, or volume). Consistent with our primary LMMs, the mixedlm function from the statsmodels package and were used. Also consistent with these, TIV and puberty stage were included as covariates.
As a final approach, we combined LASSO regularization with hierarchical regression. We first constructed a baseline model for each brain outcome by regressing the outcome on covariates only (TIV, puberty stage, and HC + /HC− group status) to estimate the variance explained by these factors (baseline R²). Subsequently, we modeled the residual variance (i.e., the portion of variance not explained by the covariates) using hormone levels and their interactions with HC + /HC− group status. To do this, we applied a LASSO regression with five‑fold cross‑validation, which shrinks uninformative coefficients to zero and selects the most explanatory variables. The incremental R² (representing the additional variance explained by the hormone measures and interactions) was calculated from this LASSO step, and the full model R² was obtained by summing the baseline and incremental R². This method was used to isolate the unique contribution of hormone measures and their interactions above and beyond the effects of the covariates.
link





More Stories
Estrogen therapy shows mixed mental health effects in menopause
Sexual Health’s Impact on Brain and Mental Wellbeing
Newsom vetoes transgender health measure, after chiding Dems on issue